Abstract
Sickle cell disease (SCD) is a genetic disorder caused by a point mutation in the β-globin subunit resulting in hemoglobin S (HbS). Following deoxygenation of red blood cells, HbS forms polymers that can promote hemolysis and the release of free heme that cause pro-oxidative and pro-inflammatory stress, vaso-occlusive pain crises, and ischemia-reperfusion pathophysiology. Heme also functions as an intracellular activator of antioxidant and globin gene expression. Heme binds to the transcriptional repressor BTB and CNC homolog 1 (BACH1), which relieves BACH1's repression of gene transcription. The release of BACH1 repression increases the binding of nuclear factor erythroid 2-related factor 2 (NRF2) to antioxidant response elements (ARE) and the cell-specific transcription of antioxidant genes such as heme oxygenase-1 (HMOX1), glutathione reductase (GR), solute carrier family 7 member 11 (SLC7A11), and NAD(P)H dehydrogenase [quinone] 1 (NQO1). We have previously shown that pharmacologic activation of the NRF2 pathway in SCD mice provides protection against heme-induced vascular occlusion, is anti-inflammatory, and decreases hepatic necrosis. NRF2 activation also promotes erythroid expression of the A-gamma (HBG1) and G-gamma (HBG2) globins, which are subunits of hemoglobin F (HbF) that replace β S-globins and thus increase HbF and decrease HbS in red blood cells. Thus, BACH1 inhibitors have the potential to increase expression of antioxidant and HbF genes and prevent or reduce SCD-related pathophysiology, resulting in reduction of hemolysis, inflammation, and vaso-occlusive pain crises. Mitobridge is currently developing ML-0207/ASP8731, a highly potent, selective small molecule inhibitor of BACH1 capable of activating the Nrf2 pathway in human and murine models and investigated the ability of ML-0207 to modulate antioxidant and anti-inflammatory genes and induce HbF in human translational cellular models and a preclinical murine model of SCD.
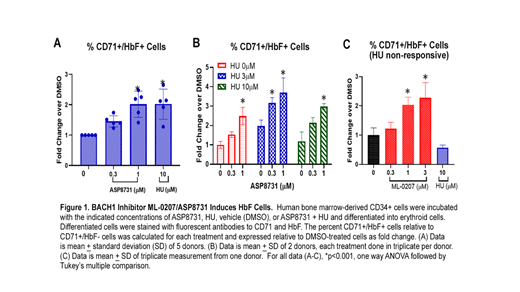
ML-0207 induced mRNA expression of Nrf2 target genes HGB1, HBG2, HMOX1, SLC7A11, GCLM, and NQO1 in human bone marrow-derived CD34+ cells differentiated to erythrocytes. We observed 2-fold increases in both the percentage and number of CD71+/HbF+ erythrocytes by FACS using 1 µM ML-0207 and 10 μM HU compared to DMSO control (Figure 1A). The combination of ML-0207 and HU induced significantly more HbF+ erythrocytes compared to each drug alone (Figure 1B). In a single healthy CD34+ donor non-responsive to 10 µM HU, we observed ML-0207 was able to significantly induce CD71+/HbF+ cells at 1 & 3 µM (Figure 1C). In Townes SCD mice, there were significant increases in heme oxygenase 1 and decreases in VCAM-1, ICAM-1, and decreases in phospho-p65 NF-ĸB protein. Furthermore, we observed a significant reduction in hemin-induced vaso-occlusion and an increase in the percentage of F-cells. The increases in F-cells were accompanied by increases in blood A-gamma globin and erythrocytes and decreases in leukocytes. Taken together, these data support BACH1 inhibitors as potential novel and effective treatments for SCD patients.
Nataraja: Mitobridge: Current Employment. Singh: Mitobridge: Current Employment. Demes: Astellas: Current Employment. Olson: Mitobridge: Current Employment. Stanwix: Mitobridge: Current Employment. Biddle: Rheos Medicine: Current Employment. Vercellotti: Mitobridge, an Astellas Company: Consultancy, Research Funding; CSL Behring: Research Funding. Belcher: Mitobridge/Astellas: Consultancy, Research Funding; CSL Behring: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal